1013Alpha Organic Chemistry Set for Student <4th Edition> + Student Guide
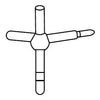
This is the organic chemistry set for student of the polyhedron molecular models. Since this set contains C(sp2), N(sp2), m(sp), S, and Cl atoms besides regular C(sp3), N(sp3), O atoms, it is possible to construct the accurate C=C double bond, benzene ring, pyridine ring, and amide group, etc. Of course, by using m(sp) atoms, it is possible to assemble the accurate C≡C triple bond.
Despite the affordable price, the 4th edition of this set contains the p-atomic orbital plates (2 green plates and 2 blue plates) and the orbital connectors (1 green connector and 1 blue connector), which are very important for studying the π-molecular orbitals of olefin compounds, e.g. HOMO (highest occupied molecular orbital) and LUMO (lowest unoccupied molecular orbital). For example, the π-bond of ethylene molecule is shown here.

In the ground state (HOMO), the p-atomic orbitals of two carbon atoms take the same sign or same color (green/green or blue/blue) as shown, because of the bonding character, where two p-atomic orbitals partially overlap each other. This overlap is represented by the orbital connector. By this π-bond, olefin compounds take planar structures, and hence there are cis- and trans-isomers. Since the orbital connectors block the rotation around the C-C σ-bond, they are useful for differentiating cis- and trans-isomers. On the other hand, in the LUMO, two p-atomic orbitals take the opposite signs or different colors (green/blue or blue/green), and hence the anti-bonding force works. Therefore, the orbital connectors are not used.
Thus, this set is highly recommended for undergraduate students studying organic chemistry.

- Regular price
- $18.00
- Regular price
-
- Sale price
- $18.00
- Unit price
- per
Couldn't load pickup availability
Contents
Atom
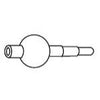
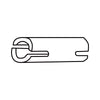
Bond(1Å = 2.5cm)
| Item No. | Bond Length (Å) | Color | Use | Qty |
|---|---|---|---|---|
| BND-02 |
1.092 1.213 |
pink
|
C-H C≡C,C=O |
30 |
| BND-04 | 1.40 |
green
|
C=C,C-O, C(ar)=C(ar) |
8 |
| BND-06 | 1.54 |
white
|
C-C,S-O | 24 |
| BND-07 | 1.80 |
yellow
|
C-P, C-Cl,C-S |
2 |
| BND-101 | 1.33 |
blue
|
C=C | 10 |
2 In the case of C-(#2 bond)-H, bond length = 1.09Å.
3 In the case of C-(#2 bond)-(non-H atom), bond length = 1.21Å.
1 Bent bond.